We are able to deliver high quality results efficiently and at a competitive price. Our team of experienced scientists, engineers, and technicians work together to provide innovative solutions for clients in the pharmaceutical business and research. Our laboratories are equipped with the latest technology and equipment, allowing us to perform a wide range of analytical tests with accuracy and precision. By owning our laboratories, we have full control over the quality and timeliness of our results, making sure that our clients receive reliable and trustworthy results every time. In addition, our laboratories overseas allow us to cut costs on R&D without loosing quality and provide us the access to biomaterial, which is more scarce in the U.S. (fresh tumor tissue).

Highly accomplished and passionate leader with 20 years of experience. Expertise in expanding market share, establishing international distribution networks, and driving significant revenue growth.Experience as Business Unit Manager at Merck and Solvay Pharmaceuticals (owned by Abbott), as well as experience at Boehringer Ingelheim.

Research scientist with 10+ years of experience in academia and industry, specializing in signaling aberrations in tumors and their microenvironment. Certified in GCP, GLP, and drug development. 15 first-author publications in top-tier scientific journals, and established partnerships with academia, biotech, and the pharmaceutical industry.

Tetiana has 8 years of project management and consulting experience. Devised and implemented corporate, marketing, and functional strategies for fast-growing small and mid-sized companies in various industries. Expertise in revenue acceleration, partnership development, client relations, and strategy ideation.

Physician scientist with more than 2 decades experience in oncology and more than 15 years of drug development expertise, including CAR T, CD3 bispecific, and ADCs. Proven leadership of large clinical development teams. Experience developing clinical strategies and acting as medical monitor - leading phase 1 and phase 1b heme and solid tumor cancer clinical trials. Ethical, collaborative, and process-driven.

Accomplished biomedical scientist with more than 15 years experience in academia and pharmaceutical industry. PhD in biochemistry and molecular biology. Postdoctoral training in pulmonary disease biology. Senior principal scientist at Bayer. Expert in discovery and validation of projects for cardiomyopathies and heart failure. Experience in disease modeling and drug discovery techniques for multiple therapeutic areas, including oncology.

Serial entrepreneur. Expert in demand generation and hyperscaling for B2B and B2C companies, including software, pharma, banking, logistics and technology. 25 years experience in operations, marketing, fundraising, business optimization and leadership of companies globally as founder, CEO, COO, CSO and CMO. Recently scaled technology company from 5m to a run-rate of 120m in 3 years. Drives company strategy, partnerships, and key operational growth initiatives in collaboration with C-level executives and growth teams.
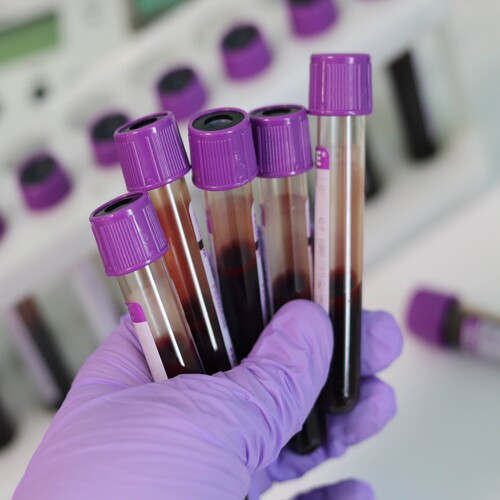
We are a clinical research organization, which provides a variety of well-characterized biomaterials for pharmaceutical companies and research organizations, including tissue samples, cell lines, and 3D models of the tumor.
Customizable solutions for clinical trials using 3D models.
Access to a large pool of biosamples, including cell lines and organoids.
Full control over product cycle: from sample collection to delivery to the client.
Faster and more efficient clinical trials using subjects with brain death.
Reliable network of labs in America and Europe for processing, testing, and quality control.
Comprehensive network of human biospecimen collection sites from North America, Europe, Asia, and Africa.